
Molecular Symmetry and point groups
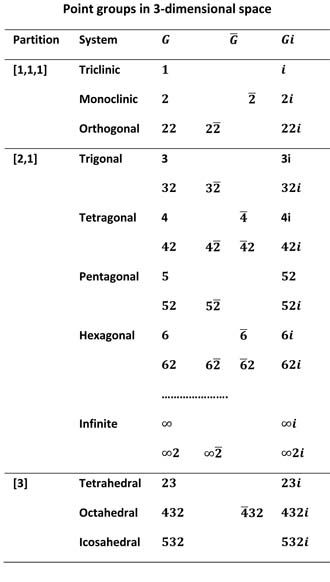
Molecular symmetry is described by the three dimensional point groups in the table on the right. Each point group in the table belongs to a spatial partition and within this division to a system based on the main axial order of the group. Systems themselves are divided into rows of Laue classes based on subsidiary axial orders that may be present. Each Laue class contains a set of groups defined by a rotational group G containing 1,2 or 3 generator rotations. The next column contains semi-direct product groups that contain the same rotational operations as the rotational group but in which one of the generators is combined with spatial inversion. The inverted generator is distinguished with an over-bar. Groups of this kind have exactly the same abstract algebraic structure as the rotational group because inversion does not change the rotational component of the multiplication product. Groups in a Laue class have the same multiplication table except that the centred group is direct product of this table. A final column contains groups Gi that are direct products of the rotational group for a given class and spatial inversion. These groups contain the space inversion operation itself and are of twice the order of other groups in the class. The order of any group is quickly found by multiplying together the numbers describing the group, counting the space inversion symbol i to be 2. so the order of 62i is 24. (does not work for group 23, a group that could be shown as 232). Images of molecules arranged in the Laue class layout allow the various operations to be pictured.
Rotational groups in the first two partitions [1,1,1] and [2,1] consist of cyclic groups n and dihedral groups n2. Cyclic groups n have just one generator, a rotation about the main axis,and repeated applications of this generator produce the n operations of the group. Dihedral groups include a second generator, a 2-fold rotation at right angles to the main axis, and combinations of this generator produce the 2n operations of these groups. Molecules of the [3] partition have multiple rotational axes that are not necessarily at right angles to each other although some are. The tetrahedral and octahedral Laue classes are related in a similar way to that of the cyclic and dihedral groups in the less symmetgric partitions. All octahedral groups have an index-2 tetrahedral subgroup that provides an underlying symmetry. Chemists have generally described molecules in the [1,1,1] partition as asymmetric tops, those in the [2,1] partition as symmetric tops while those of [3] partition are called spherical tops.
Irreducible representations
Symmetry operations in a three dimensional space can be described by matrices showing how atoms are exchanged during the operation. A set of i symmetry equivalent atoms is described by a set of i x i square matrices. Permutation representations produced in this way can always be reduced to a number of 1, 2 and 3 dimensional matrices called irreducible representations (irreps). Representations of this kind are important in describing the phyusical properties of molecules. Group representations in the [1,1,1] partition can be reduced to a number of 1-dimensional irreps. Representaions of [2,1] groups reduce to 1 and 2 dimensional irreps while those of [3] groups reduce to 1,2 and 3 dimenisional irreps. Molecular irreps are easily found from a number of simple rules.
Groups in a Laue class have the same irreps because irreps are decided by the abstract structure of the group and groups in a Laue class have the same underlying structure. Direct-product (centred) groups are direct products of the defining Laue class group and the the abstract 2-fold group. Irreducible reps for the centred group are therefore just products of the Laue class irrep and a 2-fold irrep. It follows that many of the necessary deductions required are the same for all members of a Laue class.
Click on a row for more information on groups in that Laue class
The notation in the table above was derived as part of a system to describe point groups in higher dimensional spaces in which the 2-fold dihedral operation was given label u. Later work revealed the advantages of this system for molecular point group applications in three dimensions. Replacing the letter u by the number 2 allows this system to appear quite similar to the three dimensional international notation, particularly for the rotational groups and there is no ambiguity in three dimensions.
Schoenflies notation applied to molecular structure
Atomic and molecular structures are usually defined with Schoenflies notation but this approach was intended to describe the external morphology of crystals. It was derived by various researchers during the 19th century and published in its final form in 1891, a quarter of a century before the advent of simple particle models of the atom. It does not work very well when applied to atoms and molecules because it is a geometrical description of the structure rather than the algebraic description necessary to handle point group properties.
Hermann-Mauguin notation
Schoenflies notation was replaced in crystallographic use following the introduction of the Hermann-Mauguin (International) system in 1931. This allowed each of the 219 three dimensional space groups to be described directly but provided no advantage for molecular work where point groups are sufficient. In fact, neither of these systems is very helpful for molecular symmetry because both are fundamentally intended for crystallographic use.
Symmetry and point group references
There exists an enormous volume of literature on symmetry groups and their applications in crystallography and molecular work.
